Zydus Cadila, a prominent pharmaceutical company based in India, has made significant strides in the battle against COVID-19 by developing ZyCoV-D, a needle-free vaccine. ZyCoV-D, the world’s first plasmid DNA vaccine, offers a breakthrough solution for painless and efficient immunization. Moreover, with the recent supply initiation to the Government of India and plans for broader availability, ZyCoV-D is poised to play a crucial role in the country’s vaccination campaign. Keep reading to know more on rajkotupdates.news : zydus needle free corona vaccine zycov d.
Introduction
The COVID-19 pandemic has necessitated the development of effective vaccines to combat the virus. Unfortunately, traditional vaccination methods involve needles, which can be uncomfortable for some individuals and pose logistical challenges.
However, Zydus Cadila, an Indian pharmaceutical company, has pioneered an innovative solution by introducing Zycov-D, a needle-free COVID-19 vaccine. In this article, we will explore the development, mechanism, efficacy, and potential benefits of Zycov-D.
Development of Zycov-D
Zycov-D is the result of extensive research and development by Zydus Cadila. The vaccine is based on a novel DNA plasmid platform, utilizing a three-dose regimen.
Moreover, the platform involves genetically engineered DNA molecules that encode specific proteins from the SARS-CoV-2 virus.
This approach stimulates an immune response, enabling the body to recognize and neutralize the virus more effectively.
Supply Commencement and Manufacturing

According to Rajkotupdates, Zydus Cadila has commenced the supply of ZyCoV-D to the Government of India from its state-of-the-art Zydus Vaccine Technology Center of Excellence in Changodar, Ahmedabad.
In addition, the company has established partnerships with Shilpa Medicare Limited, a contract manufacturing organization, for large-scale vaccine production.
Through automation and advanced manufacturing processes, Zydus ensures the efficient production of the needle-free DNA plasmid vaccine.
Needle-Free Administration and Dosage Schedule
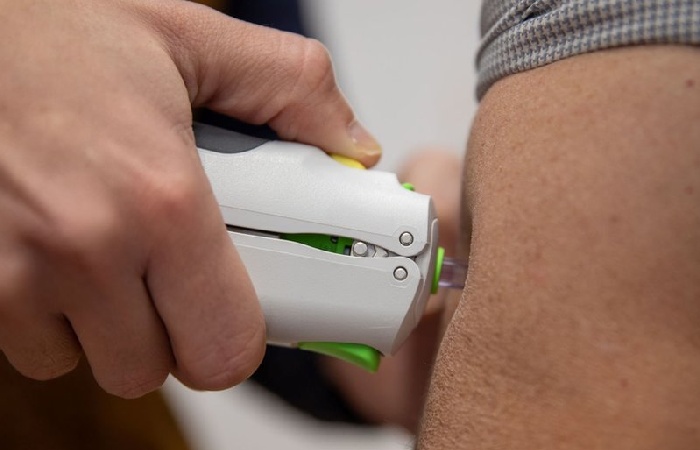
ZyCoV-D distinguishes itself with its needle-free administration, providing a painless and convenient vaccination experience. The vaccine delivers intradermally on a three-dose schedule, with doses administered on days 0, 28, and 56.
In addition, the innovative PharmaJet needle-free system, known as Tropis, facilitates the intradermal delivery of the vaccine, eliminating the need for traditional needle injections.
Efficacy and Safety
Zycov-D has undergone extensive clinical trials to evaluate its safety and efficacy. The results from phase 1 and 2 trials have shown promising outcomes, with the vaccine demonstrating good immunogenicity and a favorable safety profile.
Phase 3 trials are underway to assess the vaccine’s effectiveness in a larger population. Preliminary data suggest that Zycov-D generates a robust immune response, including the production of neutralizing antibodies, and exhibits potential efficacy against different variants of the SARS-CoV-2 virus.
Cost and Accessibility
Zydus Cadila has set the price of each ZyCoV-D dose at ₹265, with an additional ₹93 per dose for the needle-free applicator, excluding GST. The affordable pricing strategy aims to ensure accessibility and affordability for the population.
In addition, by partnering with Shilpa Medicare Limited for manufacturing and distribution, Zydus Cadila aims to meet the demand and make ZyCoV-D available in the market.
Potential Benefits
The introduction of Zycov-D brings several potential benefits to the vaccination landscape. Firstly, the needle-free delivery system reduces the fear and anxiety of needle injections, making the vaccine more accessible and appealing to a broader population. Moreover, this method simplifies administration, enabling mass vaccination campaigns and reaching remote areas more efficiently.
Additionally, the DNA-based platform of Zycov-D offers certain advantages. The production process is relatively faster and can scale up compares to traditional vaccine manufacturing methods. Furthermore, the stability of the DNA plasmids allows for storage at standard refrigeration temperatures, eliminating the need for ultra-cold chain storage and transportation infrastructure.
Regulatory Approval and Distribution Strategy
ZyCoV-D has received restricted emergency use approval from the National Medicines Regulator for individuals aged 12 and above.
The Union Health Ministry has devised a distribution strategy prioritizing districts with low first dose coverage in Bihar, Jharkhand, Maharashtra, Punjab, Tamil Nadu, Uttar Pradesh, and West Bengal.
This targeted approach aims to enhance vaccination coverage in the most needed areas. Following the initial phase, the vaccine will roll out nationwide to strengthen the immunization drive further.
Global Impact and Challenges
Zycov-D has the potential to make a significant global impact by contributing to the ongoing efforts to control the COVID-19 pandemic.
Its needle-free delivery system and other advantages make it a valuable tool, particularly in regions with limited healthcare infrastructure or vaccine hesitancy.
However, regulatory approvals, large-scale production, and equitable distribution must address to ensure widespread access to this innovative vaccine.
Partnerships and Future Prospects
Zydus Cadila has established strategic collaborations to enhance the production, licensing, and technology transfer of ZyCoV-D.
For example, the partnership with Enzychem Lifesciences of South Korea highlights the global potential of the vaccine.
These collaborations enable Zydus Cadila to scale up production and ensure the broader availability of ZyCoV-D to meet the country’s vaccination needs.
Conclusion
Lastly, Zydus Cadila’s needle-free COVID-19 vaccine, ZyCoV-D, represents a significant milestone in India’s fight against the pandemic. With its innovative plasmid DNA technology and painless intradermal administration, ZyCoV-D offers a practical and accessible immunization solution.
The commencement of supply to the Government of India and plans for broader distribution highlight Zydus Cadila’s commitment to supporting the nation’s vaccination drive. As ZyCoV-D becomes an integral part of the immunization strategy, it holds the potential to make a substantial impact in curbing the spread of COVID-19 and protecting public health. Know more on rajkotupdates.news : zydus needle free corona vaccine zycov d.
Key Takeaway
- Zydus Cadila’s Covid vaccine, ZyCoV-D, has received authorization for emergency use from the Drug Controller General of India.
- ZyCoV-D is a needle-free vaccine administered using The PharmaJet, a needle-free applicator that ensures painless intradermal vaccine delivery.
- 60 Lakh Doses Of Zydus Cadila’s Vaccine Ready After Production In October
- Zydus Cadila’s ZyCoV-D To Be Introduced In Covid Vaccine Drive Shortly
- Zydus Cadila’s Covid Vaccine ZyCoV-D To Be Used In 7 States Initially
- Drugmaker Shilpa Medicare Limited Produce Pharma Firm Cadila’s 3-Dose COVID-19 Vaccine ‘ZyCov-D
- Zydus Cadila Begins Supply Of Covid Vaccine ZyCoV-D To Government
- Central Drug Regulator Approves Shelf Life Of COVID-19 Vaccines CoviShield, Covaxin, ZyCoV-D
- India To Buy 1 Crore Zydus Cadila ‘Needle-Free’ Covid Shots At Rs 265 Each
- Zydus Cadila Agrees To Reduce Covid Vaccine Price To Rs 265 Per Dose
FAQS
Q: What is ZyCoV-D?
A: ZyCoV-D is a needle-free COVID-19 vaccine developed by Zydus Cadila, the first plasmid DNA vaccine in the world.
Q: How is ZyCoV-D administered?
A: ZyCoV-D is administered intradermally using a painless needle-free applicator called PharmaJet, delivering the vaccine in three doses on days 0, 28, and 56.
Q: Has ZyCoV-D received regulatory approval?
A: ZyCoV-D has received restricted emergency use authorization from the National Medicines Regulator for individuals aged 12 and above.
Q: Where is ZyCoV-D being manufactured?
A: ZyCoV-D manufacture at Zydus Cadila’s Zydus Vaccine Technology Center of Excellence in Changodar, Ahmedabad, India.
Q: What is the cost of ZyCoV-D?
A: ZyCoV-D is ₹265 per dose, with an additional ₹93 per dose for the needle-free applicator, excluding GST.
Q: Which states in India will initially receive ZyCoV-D?
A: ZyCoV-D will be rolled out in Bihar, Jharkhand, Maharashtra, Punjab, Tamil Nadu, Uttar Pradesh, and West Bengal.
Q: How many doses of ZyCoV-D are required?
A: Three doses of ZyCoV-D are required, with a 28-day interval between each dose.
Q: Is ZyCoV-D available for all age groups?
A: ZyCoV-D has received approval for individuals aged 12 and above.
Q: Are there any manufacturing partnerships for ZyCoV-D?
A: Zydus Cadila has partnered with Shilpa Medicare Limited for manufacturing and Enzychem Lifesciences of South Korea for product licensing and technology transfer.
Q: What is the significance of ZyCoV-D being a plasmid DNA vaccine?
A: ZyCoV-D, a plasmid DNA vaccine, offers the advantage of direct genetic material delivery into cells, potentially inducing a robust immune response against COVID-19.
Related Searches to rajkotupdates.news : zydus needle free corona vaccine zycov d
rajkotupdates.news : zydus needle free corona vaccine zycov d
free coronavirus
rajkotupdates न्यूज़ zydus सुई फ्री कोरोना चेचक वैक्सीन zycov d
ज़ीकोव-डी
covid vaccine patna
zycov-d vaccine
zycov d vaccine news
vaccine bihar
vaccination patna
bihar vaccination news
vaccination in bihar
corona vaccine free in india
bihar vaccine update
how to register for covid vaccine in bihar
covid vaccine bihar
zycov d vaccine launch date
vaccine in bihar
zycov vaccine
free corona vaccine in india
rajkotupdates news zydus सुई मुक्त कोरोना चेचक वैक्सीन zycov d
bihar vaccine





One Comment
[…] SUPER LOTTO with all date and time details. It’s one of the most accurate lottery results & news providers of different national and international […]